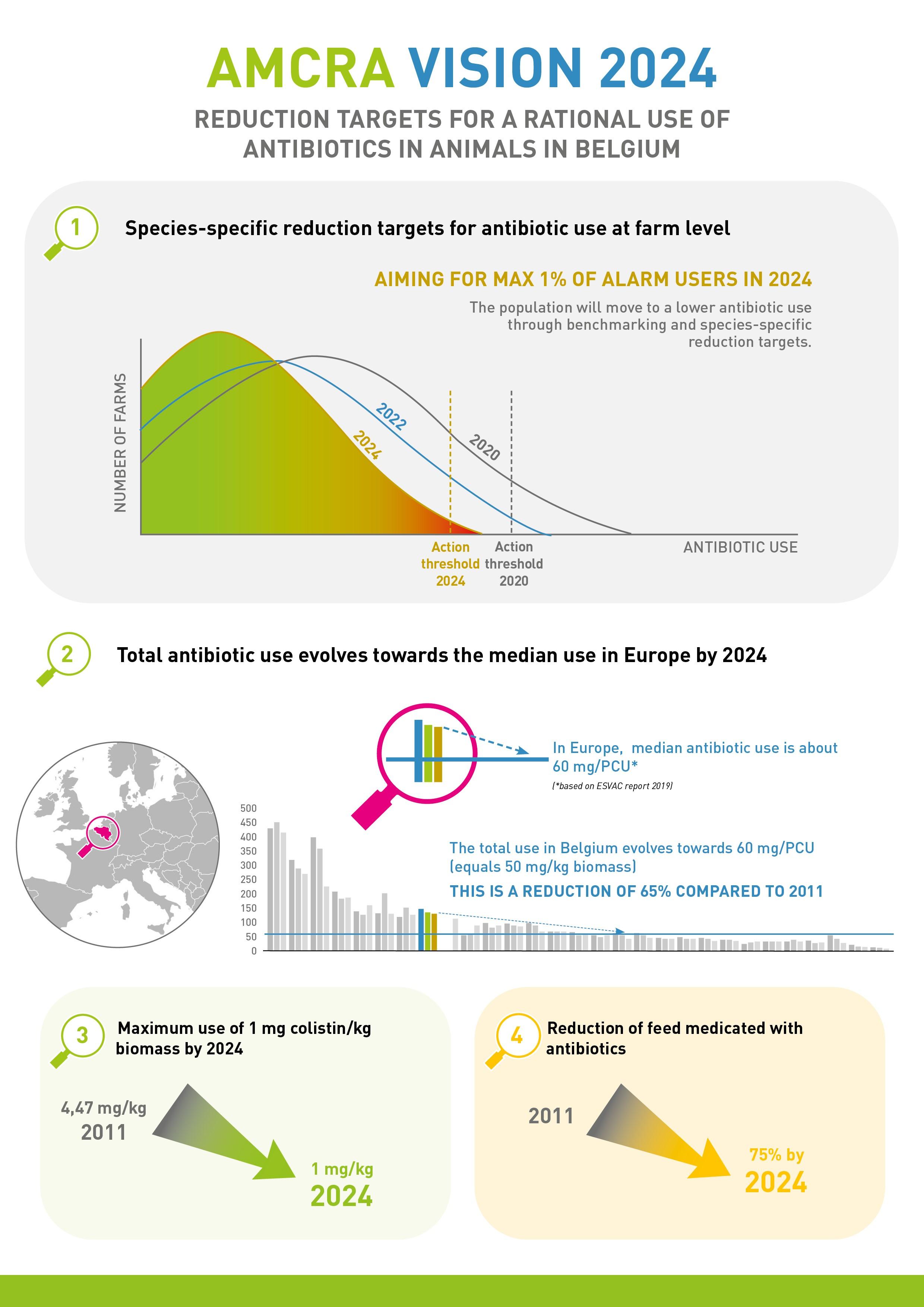
The AMCRA Vision 2024 was developed in 2019 by the members of AMCRA to set out its ambition on a more detailed antibiotic policy in relation to animals in Belgium following the end of the first reduction plan in 2020.
The AMCRA vision 2024 contains four strategic objectives relating to the policy on antibiotic use and resistance in animals in Belgium between 2021 and 2024, which are linked to nine action points.
The goal is reduce antibiotic use in all animal species and by all veterinarians to a minimum. To this end, the limits of what constitutes responsible use have been defined.
The four objectives were set based on the assumption of achieving the objectives described in the AMCRA vision 2020. Continuous monitoring of these objectives (total use, antibiotic-medicated feeds and use of antibiotics with a red AMCRA code) is intended to ensure that the defined levels are never exceeded again.
Four objectives
1. Species-specific limit values at farm-level and no more than 1% 'alarm users' by 2024
Benchmark values have been defined for each animal category (food-producing animals). This means it is clear from the outset what the limit values are for low users (green zone), intermediate users (yellow zone), and high users (red zone) in 2024. The limit values for 2024 are defined in such a way that achieving them is equivalent to the intended target of 50 mg/kg biomass across all species. These values were established in 2020 on the basis of the benchmark data available at that time. Progress towards these goals is gradual, however, and interim limits have been defined. The aim is to achieve a proportionate distribution of the burdens across the different species. This means that all livestock farmers and veterinarians are encouraged to take responsibility at the individual level.
The aim in each animal category is to maximise the numbers of low users and minimise the number of potential intermediate and high users. High users (red zone) are actively traced and contacted. Farms and animal categories in the red zone are required to draw up a plan together with the farm vet, which stipulates the actions that are needed in order to significantly reduce antibiotic use. Farms and animal categories that continue to operate in the red zone in the long term or repeatedly fall into that zone are regarded as 'alarm users' (purple code). Decision trees have been created for the purpose of identifying these purple-coded farms and animal categories. Measures will also be proposed for farms and animal categories that continue to operate in the red zone in the long term. They are specially monitored by means of standards and/or through authorities. The goal is to achieve a situation in which 'alarm users' account for no more than 1% in each animal category.
The Vision 2024 defines limit values for antibiotic use in pigs, veal calves and broiler chickens at farm level. It is therefore already clear what the limit values will be for low users (green zone), intermediate users (yellow zone), and high users (red zone) in 2024.
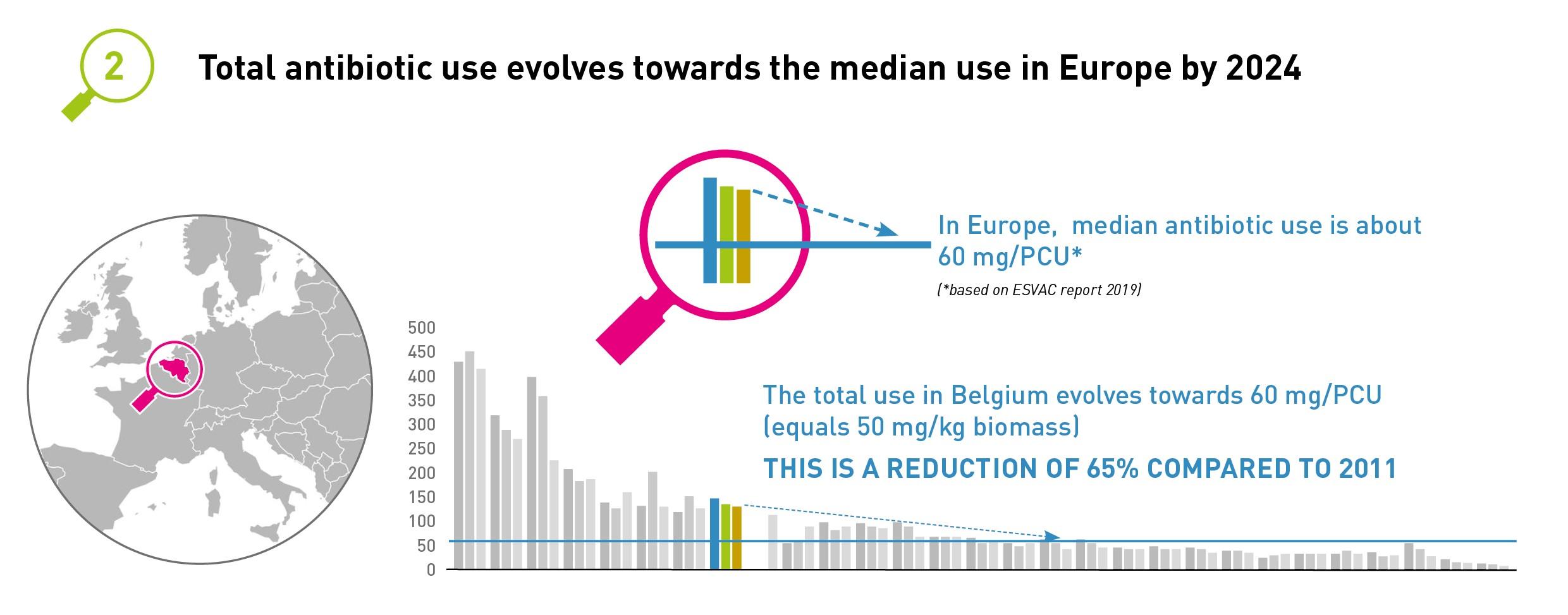
2. Total antibiotic use in animals in Belgium will progress according to median use in Europe until 2024.
The most recent ESVAC report placed the median of antibiotic use in 30 European countries at 57 mg/PCU (i.e. about 50 mg/kg biomass). Over the last few years, this median has remained relatively stable. Even countries with similar, intensive production systems such as Belgium have antibiotic use of around 50 mg/kg biomass. A gradual reduction in antibiotic use in Belgium must ultimately result in total use of around 50 mg/kg biomass by the end of 2024. This means that we would achieve a 65% reduction in veterinary antibiotic use compared with 2011.
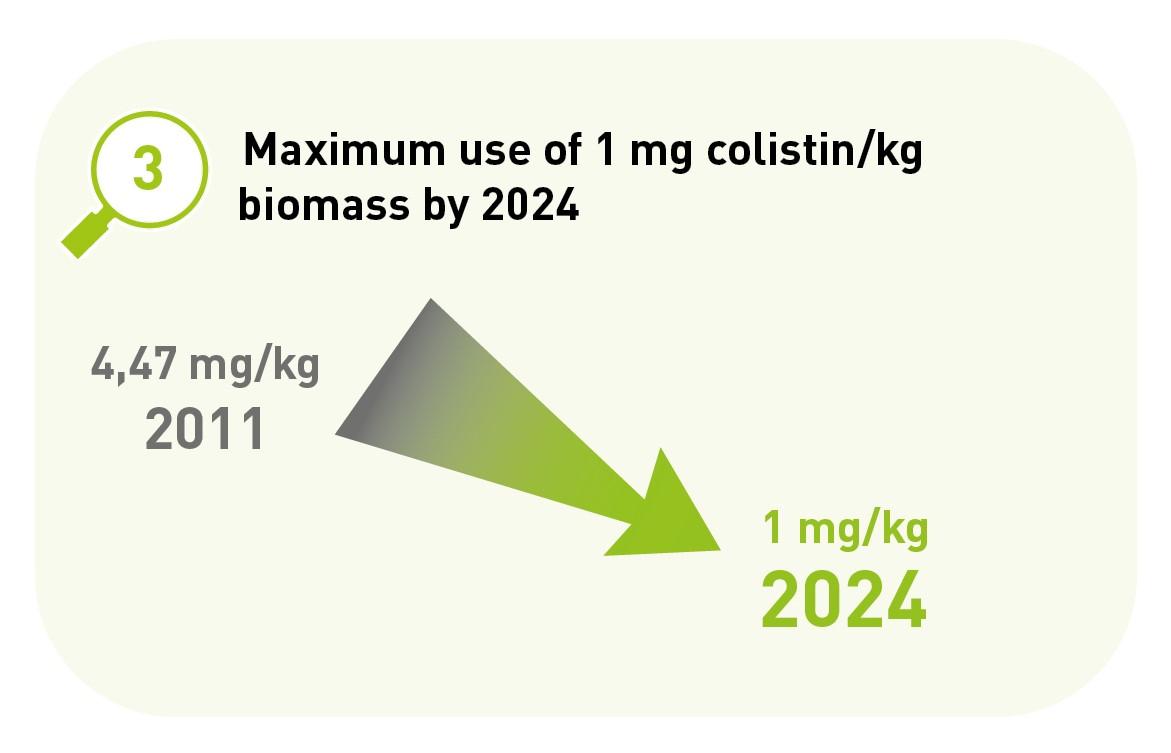
3. Maximum use of colistin is 1 mg/kg biomass by 2024.
For EU countries with low use of colistin in veterinary medicine, the EMA has assumed a use target of 1 mg/PCU. In 2018, use of colistin in Belgium was 1.69 mg/kg and had already been reduced by 64.4% since 2011. The aim is to achieve the EMA target by the end of 2024 at the latest. To this end, the use of colistin in medicated feed has been discontinued in 2021.
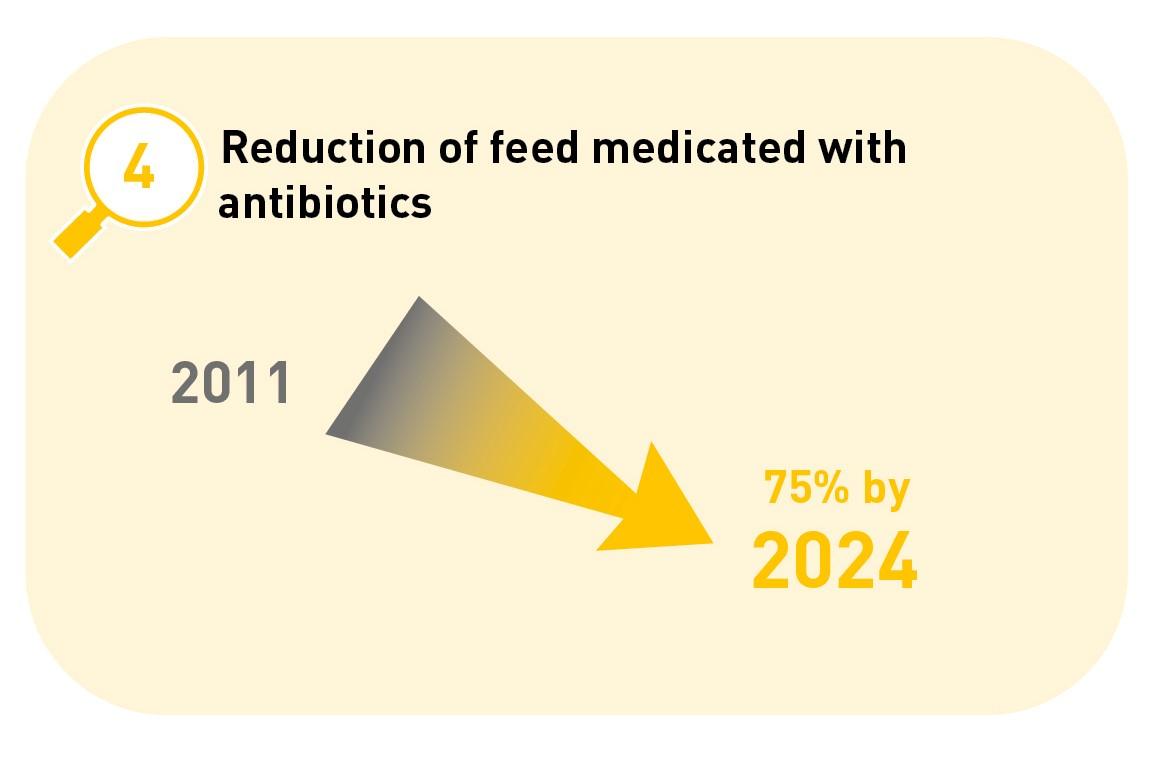
4. A 75% reduction in medicated feed containing antibiotics compared with 2011.
Nine action points
In order to achieve these targets, 9 action points have been laid down in the AMCRA Vision 2024.

At present, data on antibiotic use at farm level is already being gathered for all farms where pigs, poultry, and veal calves are being reared and at the majority of dairy farms. The aim is to continue to expand this system in order to include all food-producing animals in a statutory, mandatory, system by no later than 2022. It is also necessary to establish a system for monitoring antibiotic use in companion animals via veterinarians. Such a system needs to be operational by 2024 at the latest. Support must be provided to farm veterinarians for the administrative work performed within the scope of data collection on antibiotic use.

All veterinarians operating in the food-producing animals sector must be benchmarked using the data collected at farm level. Veterinarians operating in the non-food-producing animals sector (including companion animals, horses, exotic pets, etc.) must report antibiotic use data at practice level. Veterinarians must receive a benchmark report twice a year and, if their use is deemed excessive, are required to prepare an action plan for improvement. This plan must be examined by the authority or a veterinarians' inspection body. Veterinarians who are found to be systematically in the red zone will be actively contacted by the competent authorities.

Intermediate (yellow), high (red) and alarm (purple) users must be actively supervised in improving animal health and reducing antibiotic use on the basis of the farm health plan. This plan must stipulate clearly how measures will be taken to reduce use to a more acceptable level and what time frame will be provided for this. The preparation of the farm health plan and the monitoring of progress must be facilitated by the farm veterinarian, and also, in the case of alarm users, by an external coach. To this end, for farms in the yellow zone, at least three meetings are arranged per year between the farmer and the farm veterinarian for which the fee is covered by the Government. A team of specialist coaches are being trained (in a similar way to the antibiotic management teams in hospitals) to support farmers and veterinarians. The work of these coaches must be supported by the Government.

Reports on the use and purchase of antibiotics allow for the detection of irregularities in relation to quantitative and qualitative use. The Government arranges targeted checks at the premises of farmers and veterinarians in order to detect anomalies. To this end, field inspectors must have sufficient knowledge of the correct application of the legislation, as well as of the content and meaning of the benchmark reports. The Government must also monitor the legitimacy of use of antibiotics that are only authorised for human use, but are used in non-food-producing animals on the basis of the cascade.

The regulations on the restrictive use of "red list" antibiotics must be extended to all species. At the same time, the availability of "yellow list" antibiotics needs to be encouraged, including for minor species. It must be possible at all times for use of non-critical key antibiotics to take priority over the "red list" antibiotics. To this end, a working group will be set up for the purpose of preparing a list of options for this in cooperation with the Government. Red list antibiotics must only continue to be administered by the veterinarian and by the owner/farmer to complete a course of treatment already started. Red list products must therefore never form part of the farmer's stock.

All farmers who have a contract (with a veterinarian) for farm assistance and consequently have the right to hold a stock of antibiotics on their farms are mandatorily required to follow training in the proper and careful use of such medicines. This training must also devote attention to opportunities for preventative measures that may help to reduce antibiotic use (see phytolicence). This training must be renewed every five years. Farmers in the green zone (low users) are granted an exemption from such training.
AMCRA will continue its efforts to advise, communicate, and raise awareness via all available channels of written and verbal communication and by means of a visible presence at trade fairs and events. The organisation will work in closer cooperation with the food processing industry and retailers. AMCRA will also develop training packages that can be used for agriculture schools, universities of applied sciences, post-academic training, and so forth. This will be done in collaboration of all of AMCRA's partners. Insights obtained from the antibiotic use data analysed by AMCRA's data analysis unit will also be utilised to the greatest possible extent.
The level of biosafety and animal disease prevention on livestock farms must be increased. To this end, active programmes are being set up in order to evaluable the biosafety and the vaccination schedule at each farm and to make adjustments where possible. The biosafety evaluation will therefore become an integral part of the farm health plan. The competent authorities must actively monitor critical factors such as population density, weaning age, minimum age for transport, and so on.
Monitoring of antibiotic resistance among indicator bacteria will be extended to non-food-producing species. Greater attention and structure will be given to the monitoring of antibiotic resistance among pathogenic bacteria originating from food-producing and non-food-producing animals. To this end, a national monitoring programme will be set up and the results of this will be widely published annually. A system will also be set up in which to actively communicate the results of antibiotic resistance monitoring and make them accessible for use by veterinarians and farmers. A network of laboratories will be created that will perform antibiotic susceptibility testing according to a harmonised procedure. In addition, all laboratories that perform antibiotic susceptibility testing for the veterinary medicine sector are required to have accreditation that is issued annually by a competent authority.